Contents>> Vol. 11, No. 2
Sustainability of Acacia catechu Forest Management for Cutch Production in Magway Region, Myanmar
Wai Phyoe Maung* and Takeda Shinya**
*ဝေဖြိုးမောင်, Graduate School of Asian and African Area Studies, Kyoto University, 46 Shimoadachi-cho, Yoshida, Sakyo-ku, Kyoto 606-8501, Japan
Corresponding author’s e-mail: waiphyoemaung.fd[at]gmail.com
![]() https://orcid.org/0000-0002-5066-7108
https://orcid.org/0000-0002-5066-7108
**竹田晋也, Graduate School of Asian and African Area Studies, Kyoto University, 46 Shimoadachi-cho, Yoshida, Sakyo-ku, Kyoto 606-8501, Japan
![]() https://orcid.org/0000-0001-7565-5202
https://orcid.org/0000-0001-7565-5202
DOI: 10.20495/seas.11.2_273
Acacia catechu (Sha)-bearing forests are the primary sources of cutch, a tannin extract from the heartwood of Sha trees. Sha forests in Myanmar are managed for cutch production, and tree harvesting for cutch is regulated by an official diameter limit (ODL, 30 cm DBH [diameter at breast height]). We explored sustainable Sha forest management for cutch production through stand inventory surveys and informal interviews with locals and forest managers. We compared Sha forests with six different official harvest histories and assessed seedlings and saplings as well as the size and species of harvested stumps and remaining trees. We found that the forest understory was disturbed by surface fire, and all Sha seedlings and saplings < 1.7 m in height showed post-fire marks. We observed a regeneration gap between 1.7 m and 2.7 m, which might indicate the flame height of the surface fire. The “illegal” harvest exceeded the official harvest; only 5% of the harvested stumps were found to be larger than the ODL. Local harvesting of cutch appeared to be limited by the stem diameter required for heartwood formation (15 cm DBH). Stump data revealed that the forests were utilized not only for cutch but also for other purposes, including fuel and timber. Despite fire and local harvesting, local forest utilization patterns appear to be reasonable, although they are illegal. Implementing fire control and community management of forests along with clear definition of property rights could help in sustainably managing Sha forests for cutch production.
Keywords: NTFPs, local diameter limit, heartwood formation, surface fire, dieback, natural regeneration, community-based management
Introduction
Understanding current forest conditions and predicting the future forest structure and growing stock are fundamental steps in sustainable forest management. However, the stand structure of selectively logged forests is relatively poorly known, especially in seasonally dry tropical forests (Becknell et al. 2012, 88), although many studies have been conducted in intact, old-growth, closed-canopy tropical forests (Houghton 2005, 947).
In managing tropical forests, selective logging is based on one universal criterion: a minimum diameter cutting limit for all commercial timber species (Sist et al. 2003). This is true in Myanmar also, where commercially valuable trees are selectively extracted (Hla Maung Thein et al. 2007; Myat Su Mon et al. 2012; Tual Cin Khai et al. 2016; Zar Chi Win et al. 2018; Tual Cin Khai et al. 2020). Timber and non-timber extraction in Myanmar is carried out mainly in production forests, an administrative category that includes reserved forests (RFs) and protected public forests (PPFs). RFs are legally protected forests; they are the best-quality and higher-commercial-value forests, in which production activities can be performed only with legal permission. PPFs are also legally protected forests, but they are of lower commercial value and more accessible, with the public having some harvesting rights (Myanmar, Forest Department 2018, 2–3; 2020, 13–14).
Besides wood and timber, Myanmar is well endowed with other forest resources, including non-timber forest products (NTFPs). NTFPs are an important income source—especially for poor households (Neumann and Hirsch 2000, 33–42; Sunderlin et al. 2005, 1387; Fukushima et al. 2011, 87–88; Ei et al. 2017, 331), but also for non-poor households (Stoian 2005). About 70% of the total population in Myanmar lives in rural areas and depends on the surrounding forests for NTFPs. The Forest Department classifies legally produced NTFPs into six major groups: (1) fiber materials, (2) edible products, (3) herbal and cosmetic materials, (4) extractive resin and oleoresin, (5) non-food animal products, and (6) other miscellaneous products. Cutch is a unique NTFP extracted from the heartwood of Acacia catechu trees belonging to the extractive resin and oleoresin group (Khin Htun 2009, 18).
A. catechu (locally called Sha) is a small to medium-sized thorny tree, up to 15 m tall and 38 cm in DBH. The sapwood, which is yellowish white to yellow, is sharply distinct from the heartwood. The heartwood is light red to reddish brown, darkening on exposure to air; it is very strong and hard (Wulijarni-Soetjipto and Siemonsma 1991, 37–41). Sha is a versatile tree. The wood makes good timber and is used for house posts, agricultural implements, wheels, etc. It is very durable and resistant to attack by termites. The wood also makes excellent firewood and is one of the best woods for charcoal. Fresh leaves and small lower branches are eaten by cattle. Sha is widely distributed in the southern Himalayas of Pakistan, northern India, and Nepal, south to Andhra Pradesh in India, and east to Myanmar and Thailand (Wulijarni-Soetjipto and Siemonsma 1991, 37–41). It is distributed throughout Myanmar, except in the most humid regions (Thein Win and Ba Kaung 2005, 285). The dry and dryish districts of Myanmar are home to Sha forests that supported a thriving cutch production industry (White 1923, 80; Thein Win and Ba Kaung 2005, 285).
Cutch is produced in India, Myanmar (Dautremer 1913, 248; Wulijarni-Soetjipto and Siemonsma 1991, 37), northern Thailand (Takeda 1990), and Bangladesh (Kabir et al. 2016). Nowadays it is used mainly for dyeing and for chewing (paan). In the past, it was also used for tanning leather and as a viscosity modifier in oil well drilling. Both crude and refined cutch and bark extracts are traditionally used in medicine, usually as an astringent for the treatment of sore throat and diarrhea (Green 1995, 37–44). Cutch has been produced in Myanmar and has been the focus of a cottage industry since before colonial rule (1824) (Nisbet 1901, 439; Bryant 1997, 92). During the colonial era (1824–1948) in Myanmar, forests were territorialized as RFs by the British government. Restricted access to RFs led to a series of conflicts between cutch producers and forest officials (Bryant 1997, 92–95). Following the British annexation of upper Myanmar in 1886, conflict spread as peasants and even swiddeners became implicated in the issue. Conflict between swiddeners and the colonial state was a by-product of the tougher rules introduced after 1889 (Bryant 1997, 92–95). The rules were strictly enforced, and many individuals were prosecuted for illegal felling of Sha. At a conference of civil and forest officials held in Pyay Township, Bago Region, in March 1896, it was agreed that the Forest Department would create RFs in the best remaining cutch tracts, village reserves would be abolished, and the remaining areas would be left to swiddeners (Bryant 1997, 92–95). Afterward, the conflict between cutch traders and workers, and the colonial state continued. As the cutch price increased and the supply dwindled, cutch traders and workers ignored the rules, and theft of trees became common (Bryant 1997, 92–95). R. L. Bryant’s (1997) investigation of the history of cutch production and regulatory issues covered only the colonial era (1824–1948) and the lower Magway Region of Myanmar. However, little is known about cutch regulation and production during the postcolonial era.
Production of a wide variety of NTFPs need not involve logging (Tani 2012, 137). However, although cutch is an NTFP, production necessarily involves logging, because trees need to be cut down to extract tannin from the heartwood. After trees are cut, the bark and sapwood are removed; the heartwood is chipped into small pieces and boiled in earthen pots to extract tannin juice. The juice is collected and further reduced by boiling in a cauldron until it reaches a jelly-like consistency. After cooling, it hardens into solid cubes or biscuit-like formations. Sha forests, logging, cutch production, and NTFPs in the Magway Region are intertwined. Although a number of case studies of NTFPs have been published, few are from Myanmar (Tani 2012, 138; Ei et al. 2017, 331). In addition, the forestry sector in Myanmar is more or less focused on teak, given its commercial importance and timber quality. Many studies conducted during the last few decades have focused on the structural and compositional aspects of teak-bearing forests (Hla Maung Thein et al. 2007; Tual Cin Khai et al. 2016), and little is known about Sha–bearing forests and their utilization. Accordingly, Sha forests should be given priority for management, utilization, and conservation initiatives. Understanding stand structure and species composition is fundamental to sustainable Sha forest management and the security of local people’s livelihoods. Therefore, we conducted stand inventory surveys with the main objective of determining the sustainability of Sha forest management for cutch production. We examined (a) the structure and regeneration of officially and locally extracted Sha forests, (b) local utilization patterns, and (c) possible implications for forest management.
Materials and Methods
Study Site
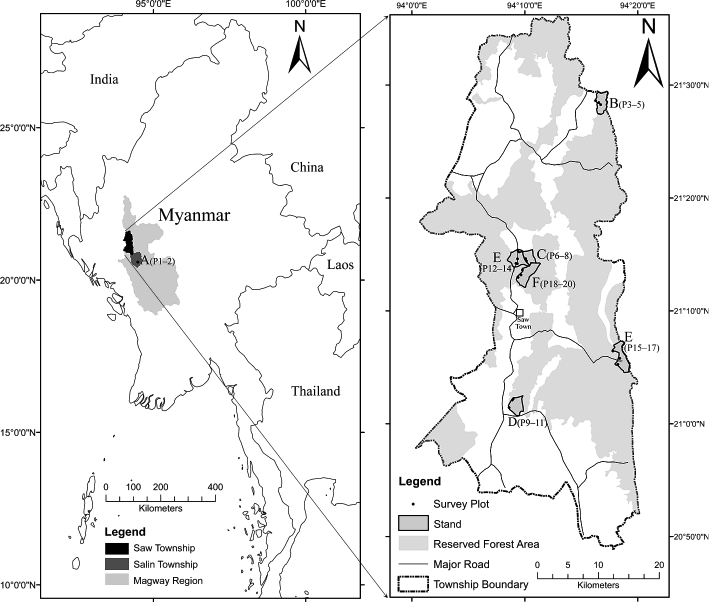
The study was conducted mainly in Saw Township, in the western part of Magway Region, central Myanmar (formerly called Burma), at 20°48′–21°20′ N, 94°00′–94°20′ E (Fig. 1). The township measures 31 km from east to west and 87 km from south to north, and has a total area of 1,779 km2. Its eastern and western parts are mostly hilly. The elevation ranges from 325 to 1,168 m a.s.l., with an average of 381 m a.s.l. The township is surrounded by six administrative townships where cutch production also occurs. Although the township lacks a major river, there are many streams and streamlets that are important freshwater sources for agriculture, cutch production, and daily use (Myanmar, General Administration Department 2017, 1–13). The climate is governed by tropical monsoon circulation. From 1999 to 2019, the mean temperature was 22.9°C and the mean annual rainfall 2,595.5 mm (Zepner et al. 2020). The major forest types are mixed deciduous (76.35%), followed by deciduous dipterocarp (17.2%), dry (4.26%), and hill evergreen forests (2.17%) (Myanmar, Forest Department 2016, 35). Hill evergreen and deciduous dipterocarp forests occupy the higher elevations, mixed deciduous forests grow at moderate elevations, and dry forests grow at lower elevations. Sha trees are found in mixed deciduous and dry forests (Thein Win and Ba Kaung 2005, 285). Saw Township contains 14 RFs and one PPF. The RFs are subdivided into compartments according to drainage and geographical situation. Those in Saw Township used to be among the best cutch-bearing production forests in Myanmar; however, since 2013 cutch production has been prohibited in Saw Township by the local government, although it is allowed in some neighboring townships. In 2018 production was permitted in neighboring Salin Township, at 20°21′–20°55′ N, 94°15′–94°50′ E.
Fig. 1 Map of Study Area and Selected Stands (A–F)
A–F indicates the stands with six official harvest histories; A = 1 year ago, B = 7 years ago, C = 9 years ago, D = 15 years ago, E = 18 years ago, and F = no official harvest since 1999. P denotes plot.
Data Collection
We made a preliminary visit to the study area in August–September 2018 and analyzed the township administration map and the cutch production history records from the local forest office. We obtained data on government regulations, local harvesting practices, and the history of each site through informal interviews with 12 individuals (one senior forest official and two forest rangers at the local office, and three cutch producers and six local harvesters in their villages). We selected Sha forest stands with six different official harvest histories (A–F) (Table 1). The time from the last official harvest of A. catechu from the forest stands varied from one year (stand A) to 18 years (stand E), while stand F had no official harvest record after 1999. Stands B–F belonged to RFs located in Saw Township and stand A to a PPF located in Salin Township (Fig. 1; Table 1). We conducted a stand inventory survey in August–September 2019 within the selected stands. We arbitrarily laid out twenty sample plots in total (two to six per stand). A concentric circular plot design with three different radii from the same center was used (3 m innermost subplot for all seedlings and saplings; 10 m middle subplot for all tree species, bamboo, and stumps; and 25 m outermost plot only for Sha trees and stumps). In the 3 m subplot, all seedlings (height ≤ 100 cm) and all saplings (height > 100 cm but DBH < 6 cm) were identified and counted. We measured the collar diameter (D0) and height of all Sha seedlings and saplings. To identify post-fire signs (burn scars, dieback) on regenerating trees, we examined the root bases of Sha seedlings and saplings ≤ 2 m height. In the 10 m plots, all tree species ≥ 6 cm DBH were tagged and identified and their DBHs and heights were recorded. Tree specimens were collected with their local names. Taxonomic identification was later confirmed against the checklist of the trees, shrubs, herbs, and climbers of Myanmar (Kress et al. 2003). All stumps of all species were tagged and measured. In the case of older stumps that were difficult to identify, we collected wood samples and identified them by examining their physical properties (color, texture, odor, and hardness). Identification was assisted by local knowledge provided by four informants (one plantation owner, one forest ranger, and two local harvesters). Bamboo species were identified, and bamboo clumps and culms were counted. A clump of ≥ 5 culms was counted as one individual clump. In the 25 m plots, only Sha trees and stumps were measured. DBH was measured at 1.3 m above ground level with a tape measure. Stump diameter and D0 were measured at ground level (zero height). To measure tree height, the distance from base to tip was measured with a pole (10 or 15 m). Trees taller than the poles were measured with a Vertex IV hypsometer (Haglöf Sweden AB).
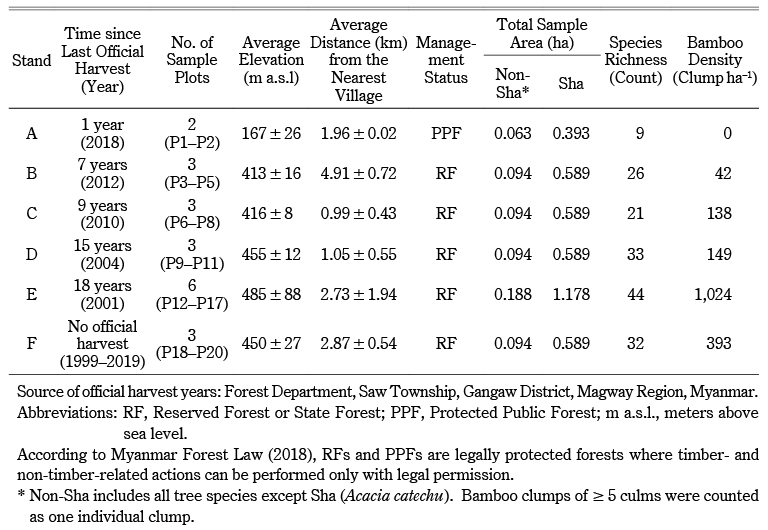
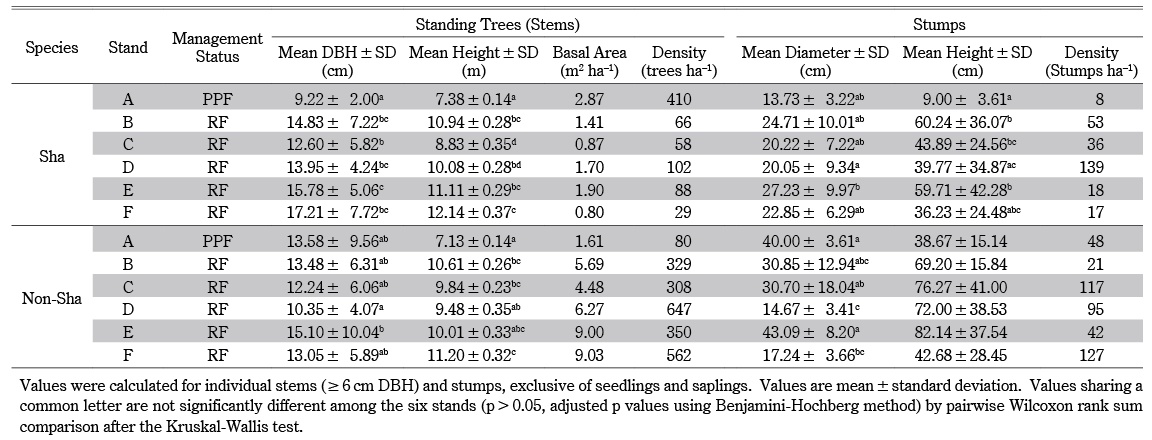
Table 1 General Characteristics of Selected Stands
Data Analysis
Sha seedlings and saplings were classified into two kinds: “with post-fire marks” and “not available (N.A.).” For all tree species surveyed, we determined the relative density [(the density of individual species/total density of all species) × 100], relative dominance [(the dominance of a species/dominance of all species) × 100], and relative frequency [(the absolute frequency of a species/total absolute frequency of the stand)]. Importance value (IV) for each tree species with ≥ 6 cm DBH was calculated as the sum of relative density, relative frequency, and relative dominance (McCune and Grace 2002, 13–23). For the vegetation structural analysis of standing trees and harvested stumps, we described, plotted, and analyzed tree and stump size data with reference to the diameter and height limits obtained in individual interviews and informal talks with the local forest office, cutch producers, and local harvesters. We did not consider dead trees or decomposed unidentified stumps. Diameters of Sha stumps were later converted into DBH using a simple linear regression model (Corral-Rivas et al. 2007, 29) based on measurements of DBH and basal diameter of ten standing Sha trees:
DBH = 0.8501 · D − 0.6983,
where DBH = diameter at breast height and D = basal diameter. Data normality was tested by the Shapiro-Wilk test and homogeneity of variance by Levene’s test. We applied the Kruskal-Wallis test to determine the effects of time since the last official harvest (categorical variable), especially on DBH and the height of Sha trees (quantitative variable) among selected stands. Additionally, we applied the Kruskal-Wallis test for diameter and height of stumps and non-Sha trees. When the results were significant, the pairwise Wilcoxon rank sum test was applied to determine which stands statistically differed from others, and P values were adjusted using the Benjamini-Hochberg method. Analyses were performed using R v. 3.6.1 (R Core Team 2019).
Results
General Forest Characteristics
The elevation of the survey plots ranged from 149 to 620 m a.s.l. The survey plots were 0.4–5.7 km from the nearest village. Stands with earlier official harvests (F, E, and D) had higher species richness. Stand A, located in the PPF, had the lowest species richness and had been harvested most recently, in 2018 (Table 1).
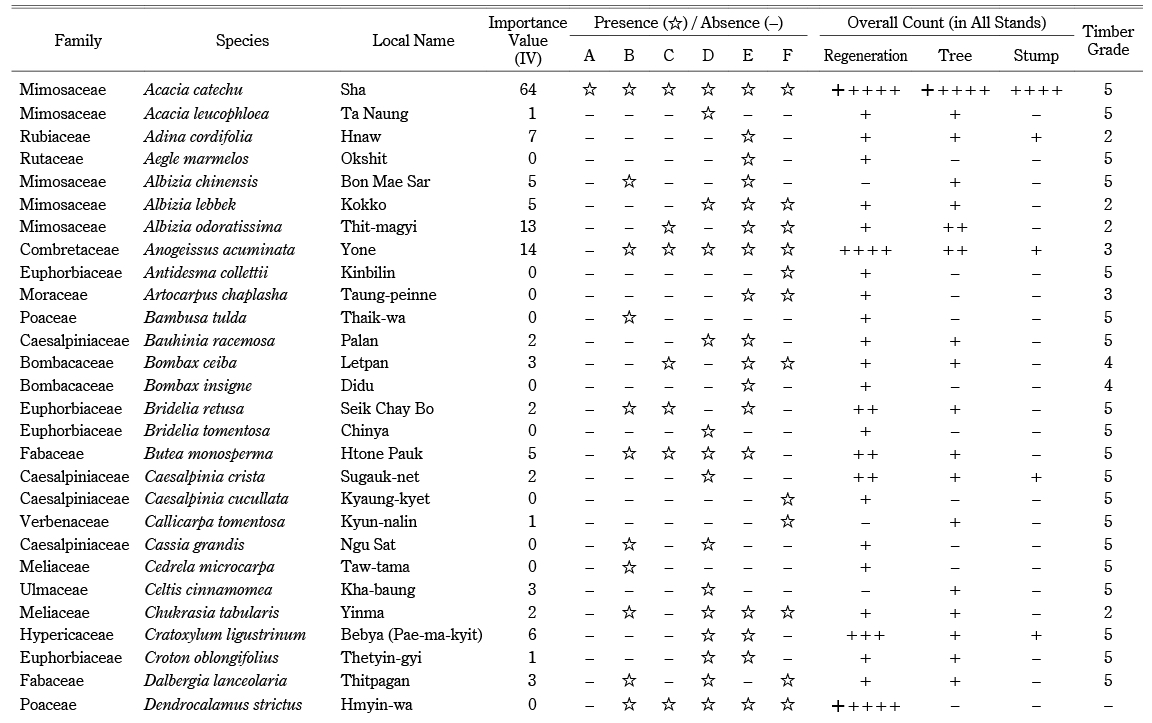
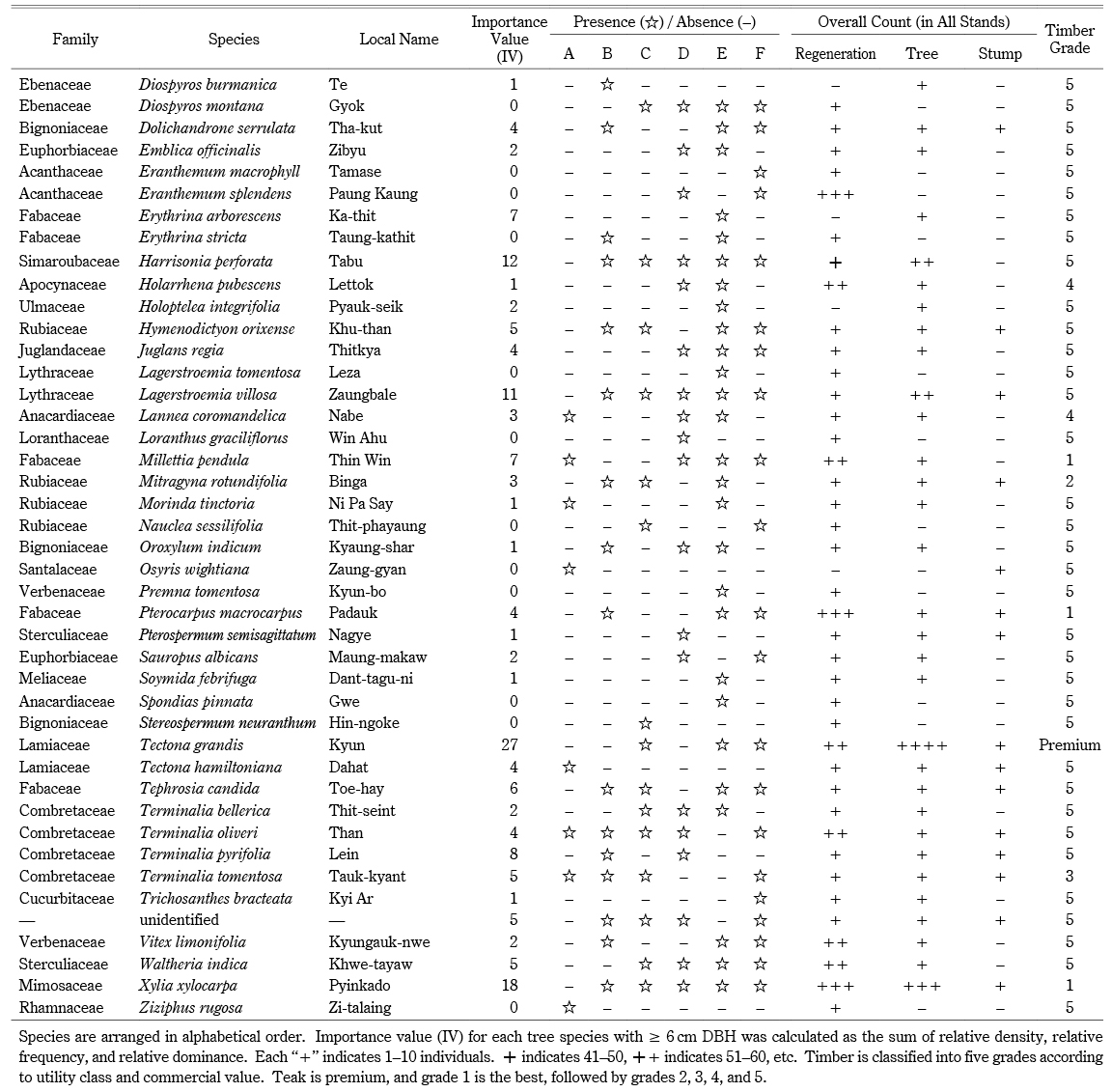
Across all stands, we identified a total of seventy species (68 tree species and two bamboo species) in 55 genera and 28 families (Appendix). The overall tree density was 496 trees ha–1. The dominant tree species included Sha (106 ha–1), Tectona grandis (49 ha–1), Xylia xylocarpa (37 ha–1), Harrisonia perforata (24 ha–1), and Anogeissus acuminata (22 ha–1). Sha trees accounted for 21% of the overall tree density. The overall bamboo density was 377 clumps ha–1. Bambusa tulda was found only in stand B; Dendrocalamus strictus was found in every stand except A (Appendix). The regeneration density, including bamboo seedlings, was 11,141 ha–1 (1,592 saplings ha–1 and 9,549 seedlings ha–1). Seedlings and saplings were dominated by Sha (1,556 ha–1), D. strictus (1,450 ha–1), H. perforata (778 ha–1), A. acuminata (619 ha–1), and Eranthemum splendens (513 ha–1). The overall tree basal area of the forest was 8.245 m2 ha–1. The basal area of Sha was 1.573 m2 ha–1, and other tree species accounted for 6.672 m2 ha–1. Sha had the highest importance value (IV = 64), followed by T. grandis (IV = 27) and X. xylocarpa (IV = 18) (Appendix). As evidence of logging, we identified harvested stumps belonging to 19 species, at a stump density of 119 stumps ha–1. The most harvested species were Sha (43 ha–1), T. grandis (11 ha–1), Lagerstroemia villosa (11 ha–1), and X. xylocarpa (6 ha–1). We also found harvested stumps of Terminalia species, used in the adulteration of cutch. The majority of stumps (43%) were Sha, followed by T. grandis (teak) and other valuable species (Appendix).
Seedlings and Saplings
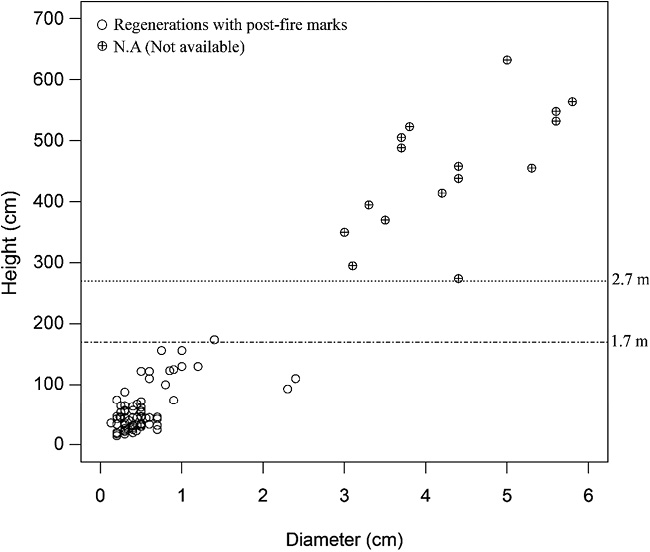
In the twenty forest understory subplots (565 m2), we recorded a total of 630 seedlings and saplings (88 Sha and 542 other species), belonging to 63 species. The regeneration density of Sha was 1,556 ha–1 (1,079 seedlings ha–1 and 477 saplings ha–1). Examining the bases of all Sha trees ≤ 2 m in height showed that all < 1.7 m in height had post-fire marks. We found a Sha regeneration gap within the height range 1.7–2.7 m (Fig. 2). In informal interviews, local people said that the forests were annually affected by surface fires in the dry season, usually from late February to April. The fires were mostly caused by anthropogenic factors—hunters, swiddeners, and honey producers—and they only burned the surface litter and undergrowth.
Fig. 2 Sha Seedlings and Saplings (n = 88) with Post-fire Marks (burn scars or bark injuries)
All seedlings and only saplings ≤ 2 m in height were examined for post-fire marks.
Diameter (cm) represents DBH for trees > 2 m and D0 for trees ≤ 2 m height.
Harvesting of Sha Trees
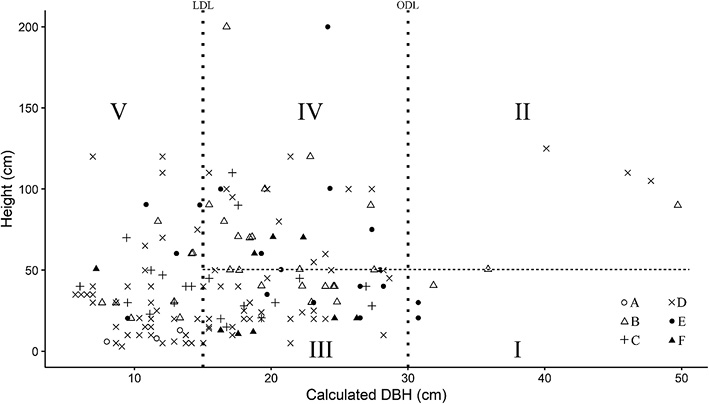
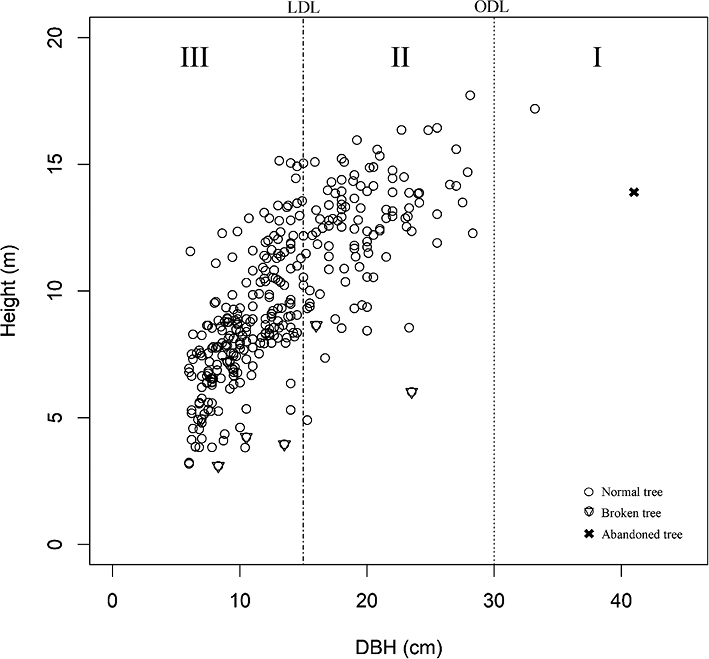
In the harvesting of Sha trees, we found two different diameter criteria and one cutting height criterion (Fig. 3). One diameter criterion was the ODL, 30 cm DBH, set by the local government to regulate cutch production. The local diameter limit (LDL) was 15 cm DBH, used by the local cutch laborers and producers, who claimed that trees of this size were harvestable as their heartwood was then well formed. As the cutting height criterion, cutch producers usually cut trees as close to the ground as possible (≤ 50 cm height) to maximize cutch yield. Thus, stumps ≤ 50 cm in height were inferred to have been harvested for cutch production.
Fig. 3 Analysis of Harvested Sha Stumps (n = 163)
Diameter criteria (LDL and ODL) are indicated by the two vertical dotted lines and the height criterion (50 cm) by the horizontal dotted line.
Stump diameter (D0) was converted to DBH. ODL, official diameter limit (30 cm DBH); LDL, local diameter limit (15 cm DBH).
Based on ODL, I + II = official harvest, III + IV + V = illegal harvest.
Based on LDL, I + II + III + IV = after heartwood well formed, V = before heartwood well formed.
Based on LDL and 50 cm height, I + III = cutch harvest, II + IV = harvest for timber, poles and agricultural tools, V = harvest for wood fuel.
A total of 168 Sha stumps were recorded in the 3.927 ha surveyed. Of these, five were too decomposed to determine whether or not they had been harvested. The other 163 stumps were partitioned into five categories (I, II, III, IV, and V) based on LDL, ODL, and cutting height (Fig. 3). A total of 5% of the stumps (eight stumps, basal area = 0.99 m2) were larger than ODL (categories I and II). The other 95% (155 stumps, basal area = 3.79 m2) were smaller than ODL (categories III, IV, and V). Mid-sized stumps, larger than LDL but smaller than ODL, accounted for 55% (90 stumps, basal area = 3.13 m2), representing trees in which heartwood had formed (categories III and IV). Stumps of trees smaller than LDL comprised 40% (65 stumps, basal area = 0.66 m2) (category V).
From LDL and cutting height, we identified different local harvest patterns. Stumps larger than LDL (categories I, II, III, and IV) accounted for 60% (98 stumps, basal area = 4.12 m2) of the total. Of these, 36% (59 stumps, basal area = 2.16 m2) were heartwood-containing trees cut at < 50 cm height (categories I and III). A further 24% (39 stumps, basal area = 1.96 m2) were heartwood-containing trees cut at > 50 cm height (categories II and IV). In total, Sha trees with a basal area of 4.78 m2 had been harvested for various purposes, including wood fuel, cutch, poles, and agricultural tools. In informal interviews with locals, we learned that smaller trees were preferable for wood fuel and charcoal because they were easier to harvest, handle, and carry. The Sha harvest rate was 1.22 m2 ha–1 in the basal area.
Structure of Stands Containing Sha
A total of 415 standing trees were recorded in the 3.927 ha surveyed. We observed seven broken or damaged tree trunks (Fig. 4). Across all stands, we observed only two mature trees larger than the ODL (category I, 3.8% of total basal area, 0.06 m2 ha–1), in stands F and B (Fig. 5). The tree in stand B was not harvested because it was infected by Ganoderma lucidum fungi: such trees cannot be used in cutch production (Troup and Joshi 1983, 17; Wulijarni-Soetjipto and Siemonsma 1991, 38). Only one other tree with a diameter > ODL was found in stand F; although this tree was a healthy one, the reason for its not being locally harvested was unknown (Fig. 5). The heartwood-containing trees that fell between the ODL and LDL accounted for 26% (108 trees) of the total standing trees and occupied 55.4% (0.87 m2 ha–1) of the total basal area. Trees smaller than the LDL accounted for 73.5% (305 trees) of the total standing trees and occupied 40.8% (0.64 m2 ha–1) of the total basal area (Fig. 4).
Fig. 4 Stand Structure of Sha Trees (n = 415) in All Periods (3.927 ha total)
Diameter criteria (LDL and ODL) are indicated by two vertical dotted lines.
ODL, official diameter limit (30 cm DBH); LDL, local diameter limit (15 cm DBH).
Based on heartwood formation, I + II = trees after heartwood well-formed; III = trees before heartwood well-formed.
I = 2 trees, 0.06 m2 ha–1 (3.8% of total basal area).
II = 108 trees, 0.87 m2 ha–1 (55.4% of total basal area).
III = 305 trees, 0.64 m2 ha–1 (40.8% of total basal area).
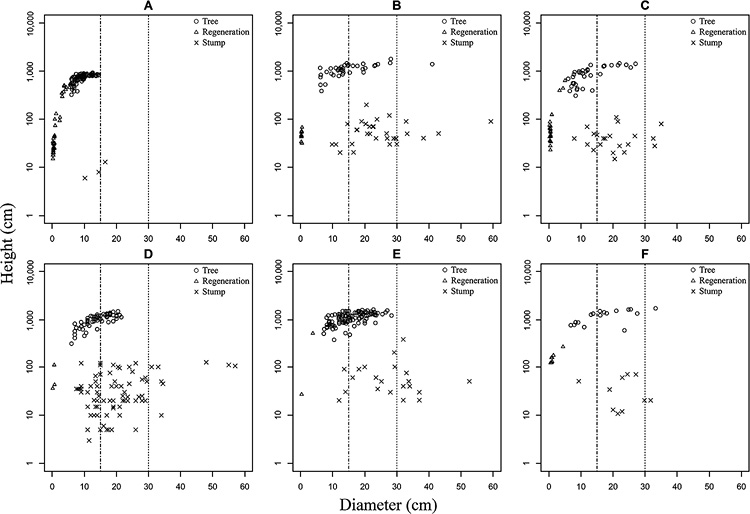
Fig. 5 Population Structure of Sha (seedlings, saplings, trees, and stumps) in Each Stand
Time since last official harvest: A = 1 year, B = 7 years, C = 9 years, D = 15 years, E = 18 years, F = no official harvest within the last 20 years.
Diameter criteria (LDL and ODL) are indicated by two vertical dotted lines.
ODL, official diameter limit (30 cm DBH); LDL, local diameter limit (15 cm DBH).
Diameter (cm) = DBH for trees and saplings > 2 m in height. = D0 for seedlings and saplings ≤ 2 m in height and for stumps.
Comparison of Vegetation Structure among the Stands with Six Official Harvest Histories
We tested the effects of time since the last official harvest (categorical variable) on the diameter of Sha trees (quantitative variable). The DBH [H = 137.43, P < 0.001] and height [H = 138.92, P < 0.001] of Sha trees differed significantly among the six stands. According to pairwise comparison, DBH and height in stand A were lowest and significantly different from all other stands (B, C, D, E, and F). The greatest DBH difference (7.99 cm) and the greatest height difference (4.76 m) were found between stands F and A. Except for stand C, which differed from E in DBH, stands B, D, E, and F, which had the same management status but different harvest histories, did not differ significantly. The tree height of stand C differed from B, E, and F, and that of stand D differed from F (Table 2). Among stands, we found significant differences in diameter [H = 19.55, P < 0.05] and height [H = 25.97, P < 0.001] of Sha stumps. According to pairwise comparison, the diameter was not significantly different among other stands (A, B, C, E, and F) except in stand D, which differed from E. The height of A was lowest among stands and significantly differed from stands B, C, and E. Stand D differed from B and E in stump height, and it had the greatest density (139 stumps ha–1) of Sha stumps (Table 2).
Table 2 Structural Attributes of Sha and Non-Sha Trees in the Six Stands
Among non-Sha trees, a significant difference in DBH [H = 13.13, P < 0.05] was observed. However, according to pairwise comparison, DBH differences were not found among the stands (A, B, C, E, and F) except in D, which differed significantly from E. The Kruskal-Wallis test showed significant height differences [H = 14.44, P < 0.05]. According to pairwise comparison, stand A was lowest and significantly different from B, C, and F. Stand D differed significantly from F (Table 2). We found significant diameter differences of non-Sha stumps (H = 23.78, P < 0.001). Stand D was lowest and significantly different from stands A, C, and E. Additionally, we found stand D had the greatest density of total (Sha + non-Sha) stumps and was second nearest to the villages (Table 1). Stand F differed significantly from A and E in stump diameter. The stump height of non-Sha species did not differ significantly (H = 8.63, P > 0.05) (Table 2).
Discussion
According to A. Raizada and G. P. Juyal (2012, 169), Sha requires strong light and does not tolerate shade during regeneration. Bamboo and teak are also light-demanding species (Hla Maung Thein et al. 2007). The dominance of light-demanding species (Appendix) at our sites might be an indicator of past disturbances in the forests. Older canopy trees may have been felled by past fire or logging, leaving gaps that light-demanding tree species could later occupy (Fig. 6). Commercial hardwood species harvested in Myanmar can be divided into five categories, based on utility class and commercial value (Appendix). Xylia xylocarpa belongs to group I, and Sha and L. villosa belong to group V (lesser-known species). Teak is the most valuable commercial timber species in Myanmar. Despite being a lesser-known species, Sha was the most commonly harvested species at our sites because of its use in cutch production. Terminalia species were also heavily harvested; in cutch production, the bark of Terminalia species is added to provide color and as a hardener (Dautremer 1913, 251). However, the locals in Saw Township reported that they did not adulterate their cutch because it reduced the quality, making it less marketable. Thus, Terminalia is likely to have been utilized for other purposes, especially for wood fuel.
Fig. 6 Photos of (a) Sha-bearing Forest Canopies, and (b) Stand Structure
In relation to forest undergrowth structure (Fig. 2), fire appears to have affected regeneration, since we found post-fire marks on seedlings and saplings, and a regeneration gap within the height range 1.7–2.7 m. These findings were supported by two studies by R. S. Troup and H. B. Joshi (1983, 10–12) and P. A. Stott et al. (1990, 32–44). According to the former, Sha seedlings have good recovery power; they die back due to fire in dry seasons and eventually shoot up when the rain falls. Thus, the majority of regenerations under 1.7 m were probably new shoots being sent up from the portions of the taproots surviving in the ground after fire. Stott et al. (1990, 32–44) have stated that fire can kill part or all of a plant, and a plant’s susceptibility to fire depends on fire intensity, length of exposure to fire, and the plant’s anatomical features, such as bark thickness and stem diameter. For example, small trees of a given species are more easily killed than large ones. The flame height of a tropical lowland forest fire can range from 0.5 to 2 m (Stott et al. 1990, 32–44). The flame height might be the threshold for seedlings transitioning into established saplings. If those seedlings grow fast enough to escape the next dry season’s surface fire, they might be able to reach the established regeneration stage.1) Otherwise, they might again be affected by the surface fire in the next dry season. The phenomenon of dying back and shooting up might be repeated until the regenerations pass the fire threshold. In this study, the regenerations we observed above 2.7 m were probably fire-resistant regenerations, and some or all of them might have been affected by fire at least once during their establishment. The linkage between our study and the above two studies by Troup and Joshi (1983, 10–12) and Stott et al. (1990, 32–44) could explain why young Sha regenerations were more vulnerable to fire and why the regeneration gap was observed. The costs and benefits of surface fire have been much debated in Myanmar (Myat Thinn 2000). Fire can harm the regeneration of some species. Suzuki Reiji et al. (2004) studied the impact of forest fires on reforestation in the Bago Region of Myanmar and concluded that such fires had a detrimental effect on the long-term sustainability of teak reforestation. However, fire can aid in the regeneration of some species by helping to break the dormancy of seeds (e.g., Sha and teak). Although Sha withstands fire well, its growth is inhibited and many young seedlings die or sustain fire damage from the annual firing of grasslands (Troup and Joshi 1983, 12). Given the regeneration gap and post-fire marks we observed, we suspect that annual surface fires hinder the regeneration of Sha.
From a policy perspective, every harvested Sha stump smaller than the ODL (Fig. 3) may be said to be “illegally” cut. In other words, the ODL can be regarded as the de jure diameter limit. From the locals’ perspective, on the other hand, this “illegal” harvest seemed rational. Because cutch is produced from heartwood, producers can harvest trees as small as 15 cm DBH, the minimum diameter for heartwood formation. In other words, a DBH of 15 cm was the de facto diameter limit (or LDL) used by local cutch harvesters. This is similar to the finding by S. S. Wanage et al. (2013) that the heartwood content of Sha trees is 40–50% once they reach 15 cm DBH. The heartwood content of trees increases in proportion to stem weight, and commercial harvesting can be initiated when trees attain a diameter of ≥ 15 cm (Wanage et al. 2013, 9). However, we found that heartwood-containing trees were not utilized solely for cutch production. Stumps with a diameter greater than the LDL and < 50 cm in height were likely to be from trees harvested for cutch production. On the other hand, stumps larger than the LDL and > 50 cm in height were likely to be from trees harvested for the manufacture of poles or agricultural implements. Sha wood is reported by locals to be durable and hard enough to be used in wooden harrows for agriculture. Sha fuelwood and charcoal are said to burn long and are thus used by blacksmiths. Stumps smaller than the LDL are likely to be from Sha trees harvested for wood fuel (fuelwood and charcoal) for two reasons: those trees could not be used to make cutch, poles, or agricultural tools; and smaller trees are convenient to cut, handle, and carry for fuel or charcoal making. There are several limitations in estimating how much wood was harvested for each purpose. We might have underestimated the harvest quantity because some stumps might have decomposed. Conversely, we might have overestimated the harvest quantity because some stumps < 50 cm in height might also have been harvested not only for cutch but also for making poles or tools. However, chances of harvesting at < 50 cm height for poles and tools are very low for two reasons: cutting at lower heights is laborious, and the amount of heartwood is relatively less important for such purposes as poles or tools.
We observed only two Sha trees larger than the ODL (Fig. 4), likely due to preferential local harvesting of young trees for cutch, wood fuel, and timber for subsistence and commercial purposes. If the largest tree had not been affected by fungi, it might also have been harvested. In fact, all Sha in RFs, regardless of size, are officially reserved by the government solely for cutch production. Nevertheless, heartwood-containing trees (category II, Fig. 4), accounting for > 50% of the total basal area, might be the de facto local reserves for future cutch production. Trees in category III (Fig. 4), which could be used for wood fuel, were developing into heartwood-containing trees. Therefore, reserving trees in category III is important for sustainable cutch production, and failing to maintain the balance between harvesting for different purposes could decrease the future cutch yield.
According to a comparison of stand structure among stands (Table 2, Fig. 5), the DBH and height of Sha species in stand A were lowest and significantly different from all the other stands; however, the DBH of non-Sha trees was not. The significant difference in DBH and height between stand A and the other stands is probably due to two reasons. First, stand A was harvested most recently (one year ago), and all remaining trees were smaller than LDL. This alternatively indicates that in stand A, it was possible that locals harvested all trees larger than LDL (Fig. 5). Second, stand A is a PPF, a forest type that is different from RFs and whose legal protection is not as strict as RFs’ (Table 2). Proximity to the villages might also be a factor affecting the significant differences in DBH and height among stands. For example, stands C and D were nearest to the villages (Table 1), and thus they had the largest density of (Sha + non-sha) stumps; and they were significantly different from stands B, E, and F (Table 2). The mean DBHs of Sha trees in stands B, D, E, and F, which had the same management status but different official harvest histories, did not differ significantly from each other. Reasonably, differences in official harvest histories signified differences in DBH among the stands if the official regulation did work. However, time since the last official harvest did not considerably affect the DBH of Sha trees, perhaps owing to local harvest patterns especially based on heartwood formation. Stump data indicated that in all stands, the official and local harvest for cutch might be synchronized2) and/or the cutch harvest is possibly accompanied by the local harvest for non-cutch purposes (Figs. 3, 5).3) Tual Cin Khai et al. (2016) conducted a field survey in teak-bearing forests and showed that repeated logging at shorter intervals could strongly degrade the forest, resulting in stands with very poor stocking, even of species with lower commercial value. They also confirmed that forest degradation was exacerbated by illegal logging, which often took place one or two years after official logging. Other field studies (Hla Maung Thein et al. 2007; Myat Su Mon et al. 2012; Tual Cin Khai et al. 2016; Zar Chi Win et al. 2018; Tual Cin Khai et al. 2020) have investigated the structure of selectively logged teak-bearing forests in Myanmar, and their conclusions converged toward deforestation and forest degradation being caused by “illegal” logging. Here, we found that the overall forest structure, though marginal, still seemed to meet local needs, because we found considerable recruitment of seedlings, saplings, and pole-size trees (Figs. 2, 4); this was also supported by the reverse J-shaped population structure (many small trees and few medium to large trees) (however, the curve is not shown in Figs. 2 and 4). Thus, from a policy perspective the forests were degrading, but from a local perspective they still seemed to meet local needs.
“Scientific” forest management in Myanmar began in 1856 with the introduction of a selection system, later accompanied by the adoption of management practices from India, which is a major producer of cutch. In India, Sha is carefully managed using silvicultural methods. In moist forests, the preferred size for cutch manufacture is 30–35 cm in diameter, with a felling cycle of ten to thirty years. In dry forests, the exploitable diameter is as low as 10 cm. Branches with a heartwood diameter of at least 2.5 cm are also used to obtain cutch (Awang and Taylor 1993, 157). The official working plan of Sha forest management in the study area prescribed selective felling of trees over an exploitable diameter, called the official diameter limit (ODL). Bryant (1998, 87) stated that the introduction of “scientific forestry” focusing on timber production while denying conventional forest use by local people during the colonial and postcolonial era led to “illegal” forest use. Illegal felling of Sha is likely to persist as long as forest management policy puts more emphasis on regulations than on local utilization and the biology of heartwood formation. At the local level, competition among different local utilization patterns (Fig. 3) might exacerbate illegal logging. This disconnect between government regulations, which are not effective at controlling logging despite state landownership, and local Sha forest users, who lack a sense of ownership, is likely to lead to resource depletion by the creation of de facto open access (Feeny et al. 1990, 8; Ostrom et al. 1999, 279). State ownership is seldom associated with successful management in less-developed countries in South Asia and Africa. In contrast, evidence is accumulating on successful community management in which users are able to restrict access to the resource and establish rules among themselves for its sustainable use (Feeny et al. 1990, 9–14). Study of these successes suggests that community management through clearly defined property rights could improve management of Sha forests for sustainable cutch production. If the local community feels that the Sha forests belong to them, they might willingly protect their own groves. Under community management, Sha forests might be controlled by a distinguishable community of interdependent users. These users might exclude outsiders while regulating use by members of the local community. They might set their own harvest diameter limits, regulations, and management plans. In this situation, local people might manage the forest by sustainably harvesting the trees to maintain a balance among utilization for cutch production, wood fuel, and timber.
Overall, we found Sha forest structure is probably shaped by two factors: multiple local utilizations (including LDL) and surface fire. Given these two limiting factors, the government should focus on promotion of community forestry, with allocation of state-owned forests as community-owned Sha forests with multiple utilization patterns. It should also provide regular or intermittent financial and technical fire control support (i.e., prescribed or controlled burning or building of fire breaks). Since sustainable forest management depends on acceptance by all stakeholders (Günter et al. 2012, 26), the government and local community should cooperatively manage Sha forests not only for cutch production but also for wood fuel and timber.
Conclusion
We examined the management of Sha-bearing forests in Myanmar from the perspectives of official regulation and local utilization. From the official perspective, Sha trees are reserved solely for cutch production, which is regulated by the diameter limit set by the government, allowing only trees exceeding 30 cm in DBH to be cut. However, our data revealed that the official harvesting regulations were outweighed by local “illegal” harvesting customs, in which the diameter limit was based on heartwood formation. As well, different local utilization purposes and patterns might exacerbate “illegal” cutting. Although cutch boilers were willing to reserve Sha trees for cutch production, other local people wanted to utilize these trees for wood fuel, charcoal production, and making agricultural implements. We conclude that Sha forest structure is probably shaped by two factors: LDL and surface fire. Further studies are required on the long-term effects of surface fire and LDL on the natural regeneration of Sha-bearing forests.
Accepted: February 3, 2022
Acknowledgments
This study was supported by grants-in-aid from the Center for On-Site Education and Research; the Graduate School of Asian and African Area Studies, Kyoto University; and JSPS KAKENHI Grant Number 20H04403. We appreciate the officials and locals from Saw Township for their assistance in field surveys. We thank the anonymous reviewers for their comments and suggestions.
References
Awang, Kamis; and Taylor, David A., eds. 1993. Acacias for Rural, Industrial, and Environmental Development. Proceedings of the Second Meeting of the Consultative Group for Research and Development of Acacias (COGREDA), Held in Udon Thani, Thailand, February 15–18, 1993. Bangkok: Winrock International and FAO.↩
Becknell, J. M.; Kissing Kucek, L.; and Powers, J. S. 2012. Aboveground Biomass in Mature and Secondary Seasonally Dry Tropical Forests: A Literature Review and Global Synthesis. Forest Ecology and Management 276: 88–95. doi: 10.1016/j.foreco.2012.03.033.↩
Bryant, R. L. 1998. Power, Knowledge and Political Ecology in the Third World: A Review. Progress in Physical Geography: Earth and Environment 22(1): 79–94. doi: 10.1177/030913339802200104.↩
―. 1997. The Political Ecology of Forestry in Burma 1824–1994. Honolulu: University of Hawai‘i Press.↩ ↩ ↩ ↩ ↩ ↩
Corral-Rivas, J. J.; Barrio-Anta, M.; Aguirre-Calderón, O. A.; and Diéguez-Aranda, U. 2007. Use of Stump Diameter to Estimate Diameter at Breast Height and Tree Volume for Major Pine Species in El Salto, Durango (Mexico). Forestry 80(1): 29–40. doi: 10.1093/forestry/cpl048.↩
Dautremer, Joseph. 1913. Burma under British Rule. London: T. Fisher Unwin.↩ ↩
Ei; Kosaka Yasuyuki; and Takeda Shinya. 2017. Underground Biomass Accumulation of Two Economically Important Non-timber Forest Products Is Influenced by Ecological Settings and Swiddeners’ Management in the Bago Mountains, Myanmar. Forest Ecology and Management 404: 330–337. doi: 10.1016/j.foreco.2017.09.015.↩ ↩
Feeny, David; Berkes, Fikret; McCay, Bonnie J.; and Acheson, James M. 1990. The Tragedy of the Commons: Twenty-Two Years Later. Human Ecology 18(1): 1–19. doi: 10.1007/BF00889070.↩ ↩
Fukushima Katsuhiro; Masuda Misa; Tani Yukako; and Shiga Kaori. 2011. The Socio-economic Role of Tree-Borne Oilseeds in Rural Livelihood: A Case Study in Karnataka State, India. Tropics 20(3): 87–95. doi: 10.3759/tropics.20.87.↩
Green, C. L. 1995. Natural Colourants and Dyestuffs: A Review of Production, Markets and Development Potential. Non-wood Forest Products 4. Rome: Food and Agriculture Organization of the United Nations.↩
Günter, Sven; Weber, Michael; Stimm, Bernd; and Mosandl, Reinhard. 2012. Linking Tropical Silviculture to Sustainable Forest Management. Bois et Forets des Tropiques 314(4): 25–39.↩
Hla Maung Thein; Kanzaki Mamoru; Fukushima Maki; and Yazar Minn. 2007. Structure and Composition of a Teak-Bearing Forest under the Myanmar Selection System: Impacts of Logging and Bamboo Flowering (Ecological Resource Use and Social Change in the Minority Regions of Myanmar). Southeast Asian Studies 45(3): 303–316. doi: 10.20495/tak.45.3_303.↩ ↩ ↩ ↩
Houghton, R. A. 2005. Aboveground Forest Biomass and the Global Carbon Balance. Global Change Biology 11(6): 945–958. doi: 10.1111/j.1365-2486.2005.00955.x↩.
Kabir, Md. Alamgir; Billah, K. M. Masum; and Parvez, Md. Masud. 2016. Acacia catechu Trees in Rice Fields: A Traditional Agroforestry System of Northern Bangladesh. International Journal of Agriculture System 4(2): 107–120. doi: 10.20956/ijas.v4i2.685.↩
Khin Htun. 2009. Myanmar Forestry Outlook Study. Asia-Pacific Forestry Sector Outlook Study II, Working Paper Series, Working Paper No. APFSOS II/WP/2009/07. Bangkok: Food and Agriculture Organization of the United Nations, Regional Office for Asia and the Pacific.↩
Kress, W. John; DeFilipps, Robert A.; Farr, Ellen; and Daw Yin Yin Kyi. 2003. A Checklist of the Trees, Shrubs, Herbs, and Climbers of Myanmar. Washington, DC: Smithsonian Institution.↩
McCune, Bruce; and Grace, James B. 2002. Analysis of Ecological Communities. Gleneden Beach: MjM Software Design.↩
Myanmar, Forest Department. 2020. Forestry in Myanmar 2019–2020. Myanmar Ministry of Natural Resources and Environmental Conservation.↩
―. 2018. Forest Law 2018. Myanmar Ministry of Natural Resources and Environmental Conservation.↩
―. 2016. Gángaw khayaing thittàw outchout-loutkainhmú simangèin ဂန့်ဂေါခရိုင်သစ်တောအုပ်ချုပ်လုပ်ကိုင်မှုစီမံကိန်း [Gangaw District forest management working plan]. Myanmar Ministry of Natural Resources and Environmental Conservation (Mimeographed).↩
Myanmar, General Administration Department. 2017. Hsàw myóne deithá hsaing’yā achet-alet myà ဆောမြို့နယ်ဒေသဆိုင်ရာအချက်အလက်များ [Saw Township profile]. Myanmar Information Management Unit.↩
Myat Su Mon; Mizoue Nobuya; Naing Zaw Htun; Kajisa Tsuyoshi; and Yoshida Shigejiro. 2012. Factors Affecting Deforestation and Forest Degradation in Selectively Logged Production Forest: A Case Study in Myanmar. Forest Ecology and Management 267: 190–198. doi: 10.1016/j.foreco.2011.11.036.↩ ↩
Myat Thinn. 2000. Forest Fire Management in Myanmar. Myanmar Forestry Journal 4: 6–12.↩
Neumann, Roderick P.; and Hirsch, Eric. 2000. Commercialisation of Non-timber Forest Products: Review and Analysis of Research. Bogor: Center for International Forestry Research. doi: 10.17528/cifor/000723.↩
Nisbet, John. 1901. Burma under British Rule and Before, Vol. 1. Westminster: Archibald Constable and Co. Ltd.↩
Ostrom, Elinor; Burger, Joanna; Field, Christopher B.; Norgaard, Richard B.; and Policansky, David. 1999. Revisiting the Commons: Local Lessons, Global Challenges. Science 284(5412): 278–282. doi: 10.1126/science.284.5412.278.↩
R Core Team. 2019. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. https://www.r-project.org/, accessed August 7, 2020.↩
Raizada, A.; and Juyal, G. P. 2012. Tree Species Diversity, Species Regeneration and Biological Productivity of Seeded Acacia catechu Willd. in Rehabilitated Limestone Mines in the North West Indian Himalayas. Land Degradation and Development 23(2): 167–174. doi: 10.1002/ldr.1067.↩
Sist, Plinio; Sheil, Douglas; Kuswata Kartawinata; and Hari Priyadi. 2003. Reduced-Impact Logging in Indonesian Borneo: Some Results Confirming the Need for New Silvicultural Prescriptions. Forest Ecology and Management 179(1–3): 415–427. doi: 10.1016/S0378-1127(02)00533-9.↩
Stoian, Dietmar. 2005. Making the Best of Two Worlds: Rural and Peri-urban Livelihood Options Sustained by Nontimber Forest Products from the Bolivian Amazon. World Development 33(9): 1473–1490. doi: 10.1016/j.worlddev.2004.10.009.↩
Stott, P. A.; Goldammer, J. G.; and Werner, W. L. 1990. The Role of Fire in the Tropical Lowland Deciduous Forests of Asia. In Fire in the Tropical Biota: Ecosystem Processes and Global Challenges, edited by J. G. Goldammer, Vol. 84, pp. 32–44. Berlin, Heidelberg: Springer. doi: 10.1007/978-3-642-75395-4_3.↩ ↩ ↩ ↩
Sunderlin, William D.; Angelsen, Arild; Belcher, Brian; et al. 2005. Livelihoods, Forests, and Conservation in Developing Countries: An Overview. World Development 33(9): 1383–1402. doi: 10.1016/j.worlddev.2004.10.004.↩
Suzuki Reiji; Takeda Shinya; and Saw Kelvin Keh. 2004. The Impact of Forest Fires on the Long-Term Sustainability of Taungya Teak Reforestation in Bago Yoma, Myanmar. Tropics 14(1): 87–102. doi: 10.3759/tropics.14.87.↩
Takeda Shinya. 1990. A Note on Catechu Production from Acacia catechu Willd. in Northern Thailand. Memoirs of the College of Agriculture, Kyoto University 136: 39–46.↩
Tani Yukako. 2012. Technical and Socio-economic Aspects of Sustainable NTFP Production: A Case Study of Thitsi Resin Production in Sagaing Division, Myanmar. Tropics 21(4): 137–160. doi: 10.3759/tropics.21.137.↩ ↩
Thein Win; and Ba Kaung. 2005. Some Prominent Tree Species of Central Dry Zone of Myanmar Characteristics and Planting Practices. Proceedings of the Annual Research Conference (Forestry Sciences), Yangon, Myanmar, 7–9 January, 2005. 2005 (ref. 13): 242–329. Yangon: Myanmar Academy of Agricultural, Forestry, Livestock, and Fishery Sciences.↩ ↩ ↩
Troup, R. S.; and Joshi, H. B. 1983. Troup’s The Silviculture of Indian Trees, Vol. 4: Leguminosae. Delhi: Controller of Publications.↩ ↩ ↩ ↩ ↩
Tual Cin Khai; Mizoue Nobuya; Kajisa Tsuyoshi; Ota Tetsuji; and Yoshida Shigejiro. 2016. Stand Structure, Composition and Illegal Logging in Selectively Logged Production Forests of Myanmar: Comparison of Two Compartments Subject to Different Cutting Frequency. Global Ecology and Conservation 7: 132–140. doi: 10.1016/j.gecco.2016.06.001.↩ ↩ ↩ ↩
Tual Cin Khai; Mizoue Nobuya; and Ota Tetsuji. 2020. Post-Harvest Stand Dynamics over Five Years in Selectively Logged Production Forests in Bago, Myanmar. Forests 11(2): 195. doi: 10.3390/f11020195.↩ ↩
Wanage, S. S.; Rane, A. D.; Gunaga, P. Rajesh; Narkhede, S. S.; and Bhave, S. G. 2013. Yield Table of Acacia catechu for the Lateritic-Humid Tropics. Journal of Tree Sciences 32(1, 2): 8–13.↩ ↩
White, Herbert Thirkell. 1923. Burma: Provincial Geographies of India. Cambridge: Cambridge University Press.↩
Wulijarni-Soetjipto, N.; and Siemonsma, J. S. 1991. Plant Resources of South-East Asia. No. 3: Dye and Tannin-Producing Plants. Bogor: PROSEA Foundation.↩ ↩ ↩ ↩
Zar Chi Win; Mizoue Nobuya; Ota Tetsuji; et al. 2018. Evaluating the Condition of Selectively Logged Production Forests in Myanmar: An Analysis Using Large-Scale Forest Inventory Data for Yedashe Township. Journal of Forest Planning 23(1): 1–8. doi: 10.20659/jfp.23.1_1.↩ ↩
Zepner, Laura; Karrasch, Pierre; Wiemann, Felix; and Bernard, Lars. 2020. ClimateCharts.net – An Interactive Climate Analysis Web Platform. International Journal of Digital Earth 14(3): 338–356. doi: 10.1080/17538947.2020.1829112.↩
Appendix List of Species with Their Importance Value (IV), Composition, and Timber Grade
Appendix List of Species with Their Importance Value (IV), Composition, and Timber Grade —continued—
1) The height growth of Sha seedlings commences when the rains start; it is vigorous during the rains and comes to a complete stop in October. Experiments at Dehra Dun, India, showed that the average height attained by Sha seedlings was 1.3 m in one growing season, 2 m in two growing seasons, and 3 m in three growing seasons (Troup and Joshi 1983, 10–12).
2) Cutch production is seasonal (usually from December to February), indicating that although they have different legal status, both official and local harvests are temporally the same. During production season local people make encampments near the streams in the forests, where both legally and illegally harvested Sha can be obtained.
3) Local (illegal) harvesting for non-cutch purposes is non-seasonal and can take place all year round.